Danaher Tests Positive for Anti-Virus Supply Chain
The curve is flattening. The shutdown phase of the COVID-19 pandemic is almost over. The next step, testing, is being queued up.
On Friday, President Trump signed a $484 billion extension to the Paycheck Protection Program (PPP). In addition to replenishing the small business loans, the new law provides $25 billion to expand testing. Things are finally starting to look positive.
But there is still one major problem.
Thus far, COVID-19 testing has been a political hot potato. Elected leaders have been unwilling to own the negative implication of confirmed COVID-19 cases. Their response? To resist scaling up testing.
Consequently, we’ve been forced to treat this like Schrodinger’s virus: Everyone must quarantine as if they’re infected and use protective measures as if they’re not. To reopen the economy safely, we need more testing to accurately determine who is infected and who is safe to be in the community.
Unfortunately, only a tiny number of businesses have the expertise to deliver high-quality tests.
According to the New York Times, The Center for Disease Control acknowledges that some tests are giving uncomfortably high rates of false negatives — meaning a patient has COVID-19 but is still testing negative. This is consistent with anecdotal evidence from New York doctors and Chinese research published at MedRxiv.
Companies charged with producing tests must be able to reduce the risk of yielding a false negative.
The other important factor is scale. Only the largest companies with the best supply chains will realistically be able to produce the huge number of tests required.
We’re talking about tens of millions of tests domestically, and that’s just the start. Many people will have to be tested several times as they return to essential public-facing jobs, like first responders, healthcare workers and delivery workers.
In a series of white papers published by The Safra Center for Ethics, a research lab at Harvard University, researchers put the daily test requirement between 2 million and 20 million.
For context, since the beginning of February, a total of only 5 million COVID-19 diagnostic tests have been performed. The new PPP funding means the pace of testing is certain to accelerate.
On Saturday, Gov. Andrew Cuomo of New York announced he is authorizing 5,000 independent pharmacies to collect COVID-19 tests. He also committed to on-demand testing for all essential workers within the Empire state, even if they don’t have any symptoms.
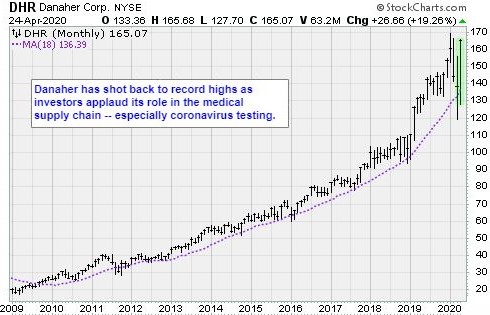
The business that’s going to reap the biggest benefits of this is Danaher (DHN).
Its wholly owned subsidiary, Cepheid, won emergency approval from the Food and Drug Administration for its COVID-19 test in March. Known as Xpress SARS-CoV-2, it’s a molecular diagnostic test that works with Cepheid’s fully automated GeneXpert system. The system consists of cartridges and an automated diagnostic machine — about the size of an inkjet printer — that can provide onsite test results in about 45 minutes.
But there’s more to this test than just speed. The real advantage of this system, according to a March press release, is that the components are already installed in 5,000 American hospitals. This foothold is the logical place to test essential workers, and it is likely to be repeated all over the country.
For Danaher, it’s a license to print money.
Danaher is a global diversified conglomerate. It operates in four major segments: life sciences, environmental and applied materials and diagnostics. The $4 billion acquisition of Cepheid in 2016 fortified its testing portfolio. It was also a great fit with the overriding corporate management philosophy.
The Washington D.C. company is built around core principles of lean manufacturing. The idea is to build synergies among its 24 subsidiaries by constantly innovating and building economies of scale. A lean, integrated conglomerate has better profit margins. It also has stronger supply chains. And in a crisis, supply chains are vital.
Cepheid is a relatively small Silicon Valley company. While its diagnostic systems are cutting edge, the testing cartridges require reagents and nucleic acids that are in short supply globally. Integrated DNA Technologies, another Danaher company, has direct access to those supply chains.
When President Trump re-upped the PPP Friday, he set the wheels in motion for the next phase of the COVID-19 pandemic response. In terms of sales, the testing phase is going to be a game-changer for diagnostic companies with proven technologies and the scale to produce tests.
Danaher shares are up 7.5% in 2020. The stock trades at 28.3 times forward earnings and 6.4 times sales. These are reasonable metrics for a well-managed company set to win big in the COVID-19 testing sweepstakes.
Re-opening the economy will only be possible with increased testing. Investors need to start looking at these kinds of opportunities with urgency. While the curve appears to be flattening, testing will be vital to make sure the trend stays that way.
Growth-oriented investors should consider accumulating Danaher shares into pullbacks.
Best wishes,